In case you missed it, we announced in July that the EspeRare Foundation and Pierre Fabre opened their clinical trial for a potential treatment of x-linked hypohidrotic ectodermal dysplasia (XLHED) in the United States. The first U.S. clinical site is at Washington University in St. Louis, Missouri.
This is great news for our XLHED families who live in the U.S. They will not have to travel to Germany to receive the treatment like Emily Nelsen and Laura Reiser did. St. Louis becomes the seventh site for the study. The study launched in Germany in November of 2021. Sites in France, Italy and Spain opened in early 2022. EspeRare and Pierre Fabre plan to open another center in the U.S. and a center in the United Kingdom will open very soon.
What The Study Hopes to Achieve
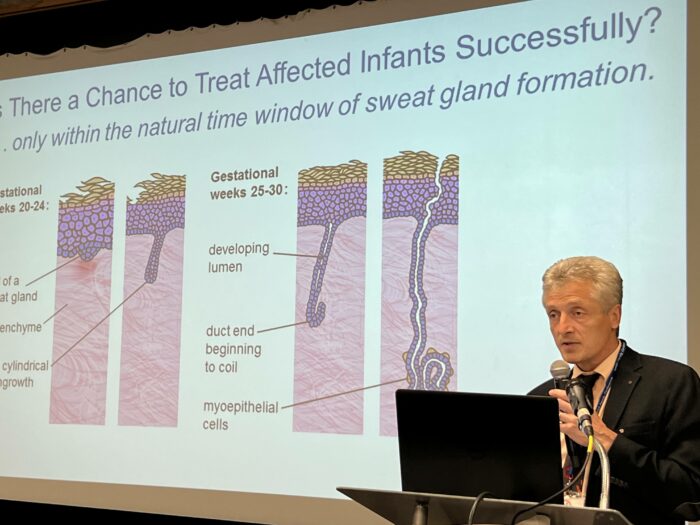
The purpose of the clinical study, called EDELIFE, is to study the safety and effectiveness of a treatment for XLHED in affected males. The study will administer a synthetic protein, ER004, to males affected by XLHED before birth, starting at the end of the second trimester of pregnancy. ER004 was created in a lab and mimics the EDA1 protein that is absent in individuals affected by XLHED. EDA1 is responsible for the development of ectodermal structures like teeth, hair, sweat glands, etc. Please visit the EDELIFE website to learn more.
The NFED is proud to work with Caroline Kant, the co-founder and CEO of the EspeRare Foundation, her team, and with the Pierre Fabre team.
It is such an exciting time for the hypohidrotic ectodermal dysplasia community. For the first time, we can hope to correct major symptoms of XLHED for those who are diagnosed before birth in the United States. We are fortunate to be able to rely on the wonderful work of the NFED to ensure that those who may benefit from this treatment (that needs to be administered before birth) are aware that the EDELIFE trial is now open in St. Louis at Washington University.”
– Caroline Kant, Founder and Executive Director, EspeRare
The trial hopes to confirm preliminary findings when three XLHED-affected boys received ER004 before birth from Dr. Holm Schneider and had improved symptoms, including the ability to sweat. According to EspeRare and Pierre Fabre, they want to 1) find out if the prenatal treatment with ER004 is well tolerated and 2) if prenatal treatment with ER004 can lead to a long-lasting improvement of some symptoms in XLHED-affected boys, including the ability to regulate body temperature.
Who’s Eligible
You are eligible for the clinical study if you are pregnant with a boy who is affected by XLHED. The study also is looking for XLHED-affected males between the ages of 6-months and 60-years-old who are blood relatives of the pregnant women participating in the clinical trial.
Researchers will compare the data collected from the babies who received ER004 before birth with the XLHED-affected males who did not receive it to evaluate and quantify the effects of the treatment.
How We Can Help
All of us at the NFED are excited to have the U.S. location open in St. Louis! Washington University is just 20 minutes from our office. If you decide to participate in the study, please email me, Mary Fete, at mary@nfed.org to let us know. We would like to support you throughout the process.
Genetic Testing Stipends
ER004 must be administered within a short window of time in the pregnancy, between 26-32 weeks with the first administration at 26 weeks. Therefore, it’s essential that women interested in participating in the study have genetic testing which documents that they are XLHED carriers. The NFED can help expedite that process by offering you a financial stipend for the testing. To learn more or apply for a stipend, email Becky Abbott, Director, Treatment and Research Advocacy, at becky@nfed.org.
Request StipendHow to Participate

Dr. Kathy Grange, a geneticist at Washington University and a member of the NFED’s Scientific Advisory Council, is the lead investigator at the St. Louis site. Contact her if you are interesting in joining the U.S. study. She can give you extensive information and can screen you for eligibility.
Dr. Dorothy Kathy Grange
Washington University
660 South Euclid Ave
St. Louis, MO 63110
grangedk@wustl.edu
314-454-6093
I am very excited to be a part of this important clinical trial that has the potential to improve the lives of boys with XLHED. Along with the rest of the team at Washington University, I am looking forward to enrolling participants at our site in St. Louis.”
– Kathy Grange, M.D., Lead Investigator
Supporting You
Of course, don’t hesitate to call me at 618-566-2020 or email me at mary@nfed.org with any questions you may have. I would love to talk to you about this important study! If you decide to participate, it is a long-term commitment, and it will require many visits to the study site. The sponsors do provide all travel arrangements and other support for your study visits. Of course, the potential improvements for your child’s symptoms could be truly life-changing!
We are working together to help you and your family participate should you choose to do so. The goal is to have this treatment available on the market by 2026 for all XLHED families. But, first the study needs to prenatally treat 15-20 boys and confirm the earlier findings.


I can’t tell you how thrilling it was to meet one of the previously treated babies, in-person! Laura Reiser came to the Family Conference last month with her one-year-old son, Bennett, and her dad, Richard Henkel, who is also affected by XLHED.
Laura shared her extraordinary story of traveling to Germany to be treated and how great Bennett is doing. He sweats! I got teary every time I saw his cute little face. He’s a miracle child, and we envision a future when this treatment may be available to all baby boys who are affected by XLHED.
Please visit our page about the clinical trial and the EDELIFE website to learn more about the trial.
